Research
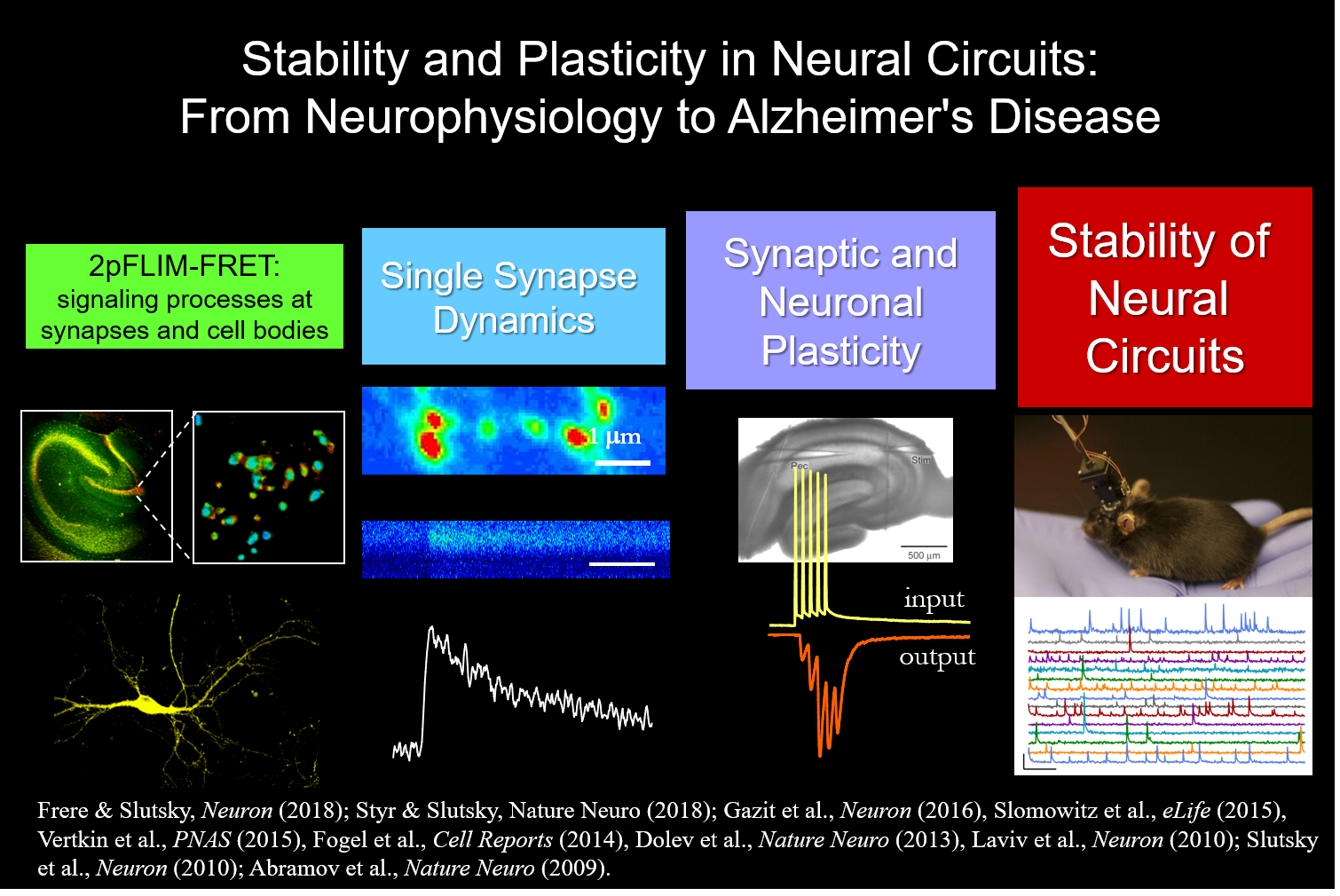
Since the early time of Descartes at the turn of the 17th century, scientists and philosophers have been searching for the physical correlate of thoughts and memories. During the past 60 years, mounting evidence indicates that experience-dependent changes in synaptic transmission and neuronal wiring, phenomena collectively termed synaptic plasticity, underlie the cellular basis of neural computation, learning and memory. Hebbian-like plasticity is crucial for refinement of neural circuits and information storage, however, alone it is unlikely to account for the stable functioning of neural networks. Both, stability and plasticity are hallmarks of brain function that enable adaptations to unpredictable and dynamic environment, experience and learning. Coping with constantly changing environments, neural circuits need to spend a considerable amount of their available energy to maintain homeostasis and to minimize the effects of stochastic events. Destabilization of hippocampal and cortical circuits has been widely documented in neurodegenerative disorders, e.g. Alzheimer’s disease, the most frequent form of late-life dementia. However, the key mechanisms that underlie stability of activity patterns in central mammalian neural circuits are largely unknown. Furthermore, how disruption of these mechanisms affects the progression of Alzheimer’s disease remains an enigma.
Our research focuses on 3 key questions:
-
How do neuronal populations achieve an ongoing balance between stability and plasticity in hippocampal circuits?
-
What are the mechanisms driving synaptic and network dysfunctions in Alzheimer’s disease?
-
What role does sleep play in the homeostatic regulation of neuronal activity?
- What are the hidden variables of population dynamics that are homeostatically regulated? What is the role of sleep in this regulation?
- Do circuits with distinct functions exhibit unique homeostatic principles?
- What are the fundamental components that form the core building blocks of the integrated homeostatic machinery crucial for maintaining stability in central circuits?
- What is the role of firing rate homeostasis in behavior?
- What role do mitochondria play in regulating stability-plasticity balance of hippocampal circuits and memory?
- How does Alzheimer’s disease impact the regulation of activity set points during low-arousal states, such as sleep and anesthesia?
- How does limbic thalamus shape synaptic, neuronal, and cognitive resilience to Alzheimer’s disease?
- Could the re-adjustment of dysregulated activity set points offer a novel approach to treating brain disorders associated with aberrant network activity?
- Calcium imaging from large-scale populations of hippocampal neurons in behaving mice using miniaturized fluorescence microscope;
- Electrophysiology: in vivo chronic extracellular single-unit/LFP/ EEG/EMG recordings; ex vivo intracellular patch-clamp recordings in brain slices and cultures, MEA (multiple-electrode-array) recordings in neuronal cultures;
- Targeted manipulations of neuronal activity using chemogenetic and optogenetic tools;
- High-resolution, quantitative imaging of synaptic vesicle recycling, calcium dynamics and mitochondrial functions;
- In vivo gene delivery using lentivirus and adenoassociated virus vectors;
- FRET: real-time imaging of inter-molecular interactions at nano-scale in live neurons;
- Molecular tools: target-specific expressing genetically-encoded fluorophore-fused proteins of interest, site-directed mutagenesis, protein knockdown.